
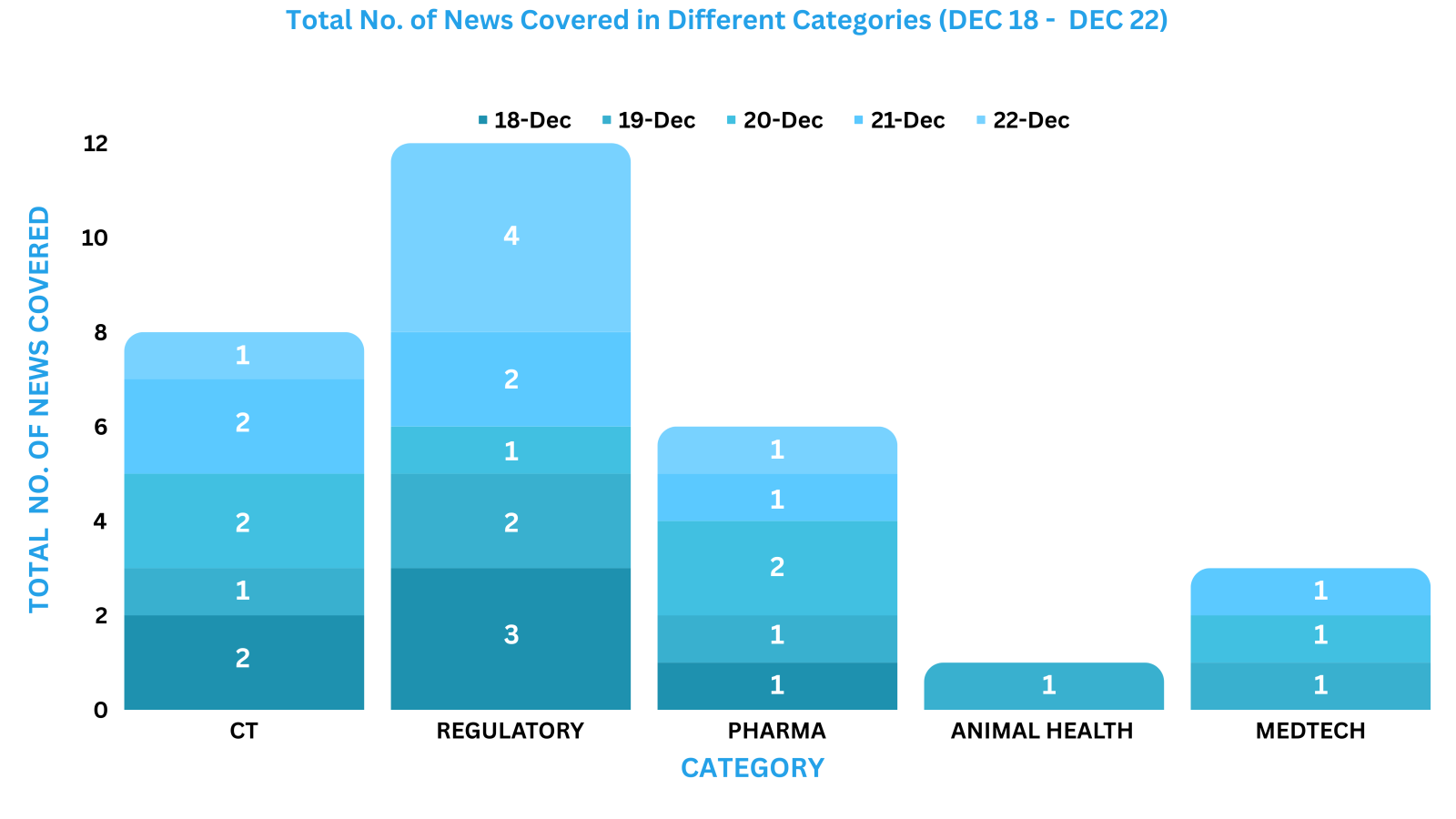
PharmaShots Weekly Snapshots (December 18 – December 22, 2023)
This week PharmaShots’ news was all about the updates on Regulatory, Clinical Trials,Pharma, Animal Health & MedTech. Check out our full report below:

The US FDA Approves Merck’s Keytruda + Padcev for the Treatment of Urothelial Cancer
Read More: Merck
The US FDA Approves Arcutis’s Zoryve (roflumilast) for the Treatment of Seborrheic Dermatitis in Individuals Aged 9 Years and Older
Read More: Arcutis
The US FDA Approves GC Biopharma’s Alyglo for the Treatment of Primary Humoral Immunodeficiency
Read More: GC Biopharma
The European Commission Extended the Approval for Ultragenyx’s Evkeeza to Treat 5 Years or Older Children with Homozygous Familial Hypercholesterolemia (HoFH)
Read More: Ultragenyx
Anheart Therapeutics & Innovent’s NDA for Taletrectinib Receives Acceptance by the NMPA and Receives Priority Review Designation for Non-Small Cell Lung Cancer
Read More: Anheart Therapeutics & Innovent
Merck’s BLA for V116 Accepted by the US FDA and Receives Priority Review for the Prevention of Pneumococcal Disease
Read More: Merck
ImPact Biotech Receives the US FDA’s Clearance on IND Application to Initiate P-I Evaluation of Padeliporfin VTP in Pancreatic Cancer
Read More: ImPact Biotech
The US FDA has granted Calliditas Therapeutics’ Tarpeyo Full Approval for IgA Nephropathy to Reduce the Loss of Kidney Function
Read More: Calliditas Therapeutics
The US FDA Approves AstraZeneca and Ionis’s Wainua (eplontersen) to Treat Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis
Read More: AstraZeneca & Ionis
The US FDA has Granted 510(k) Clearance to Alcresta Therapeutics’ RELiZORB Digestive Enzyme Cartridge
Read More: Alcresta Therapeutics
The US FDA Grants RMAT Designation to 4DMT for 4D-150 to Treat Wet Age-Related Macular Degeneration
Read More: 4DMT
Janssen Report the Marketing Authorization Application to the EMA for Lazertinib Plus Rybrevant to Treat Lung Cancer
Read More: Janssen

Innovent Highlights the Results of P-II Study for Mazdutide (IBI362) in Chinese Patients with Overweight or Obesity
Read More: Innovent
Innocare Highlights the Results of P-II Study for ICP-332 in Patients with Atopic Dermatitis
Read More: Innocare
Neuren Highlights the P-II Trial Results for NNZ-2591 to Treat Children with Phelan-McDermid Syndrome (PMS)
Read More: Neuren
Aldeyra Therapeutics Highlight the P-II Trial Results of ADX-629 for the Treatment of Atopic Dermatitis
Read More: Aldeyra Therapeutics
GSK Highlights Data from the P-III (RUBY) Trial of Jemperli (dostarlimab) + Zejula (niraparib) for Endometrial Cancer
Read More: GSK
Sanofi Discontinues its P-III (CARMEN-LC03) Trial of Tusamitamab Ravtansine for Non-Squamous Non-Small Cell Lung Cancer
Read More: Sanofi
Immunovant Highlights the P-II Trial Results of Batoclimab for the Treatment of Graves’ Disease
Read More: Immunovant
Bausch Health Reveals P-II Trial Results of Amiselimod for the Treatment of Ulcerative Colitis
Read More: Bausch Health

GQ Healthcare Entered into a Strategic Collaboration with BioMap to Co-Develop FIC/BIC ADC Therapeutics
Read More: GQ Healthcare & BioMap
Ionis and Otsuka have Collaborated to Develop and Commercialize Donidalorsen for Hereditary Angioedema (HAE) in Europe
Read More: Ionis & Otsuka
Gilead and Compugen have Collaborated to Develop COM503, a Novel Pre-Clinical Immunotherapy Program
Read More: Gilead & Compugen
Biocytogen and Neurocrine Biosciences have Entered into a Multi-Target Antibody Agreement
Read More: Biocytogen & Neurocrine Biosciences
GSK and Hansoh Pharma Enter into a Collaboration Agreement for HS-20093 to Treat Solid Tumors
Read More: GSK & Hansoh Pharma
Fauna Bio Enters into a Strategic Collaboration Agreement with Eli Lilly to Discover Obesity Targets through Convergence AI Platform
Read More: Fauna Bio & Eli Lilly

IntelGenx and Covenant Animal Health Collaborate to Develop and Manufacture VetaFilm-Based Products for Animal Use
Read More: IntelGenx & Covenant Animal Health

Koning Health Enters into a Strategic Collaboration with Gentle Scan Health to Enhance Breast Cancer Detection Across the United States
Read More: Koning Health & Gentle Scan Health
The US FDA Grants 510(k) Clearance to Perfuze’s Novel Neurovascular Aspiration and Access Catheters for the Treatment of Strokes
Read More: Perfuze
AXIM Biotechnologies Collaborates with Auer Precision for the Production of T-POC TOTAL IgE Immunoassay and T-POC LACTOFERRIN Immunoassay
Read More: AXIM Biotechnologies & Auer Precision
Related Post:- PharmaShots Weekly Snapshots (December 11 – December 15, 2023)
Tags

Kritika is a content writer at PharmaShots. She is interested in covering recent innovations from the pharma & MedTech industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.














